Long ago, the Apostle Paul said in his letter to the church in Rome that God’s creation was not only seen, but clearly seen.
In those days, there was obviously not the technology or equipment available to us in the 21st century. Still, people in the first century could see the case for creation in the cosmos1 and in the design of our body.2 That was enough. Paul said they were without excuse.
Today, scientists can peer into the very depths of the individual cell to see fascinating submicroscopic structures and their functions. Typical textbooks describing cellular anatomy and its complex processes easily exceed 1,400 pages,3 and this knowledge will only increase as research continues. With such discoveries, mankind is truly without excuse!
It has been known for decades that people, plants, animals, and bacteria have molecular machines made of protein. For example, there are such machines called repair enzymes that are currently going up and down the strands of your DNA molecule, repairing lesions (trauma) via a host of other support enzymes.4,5
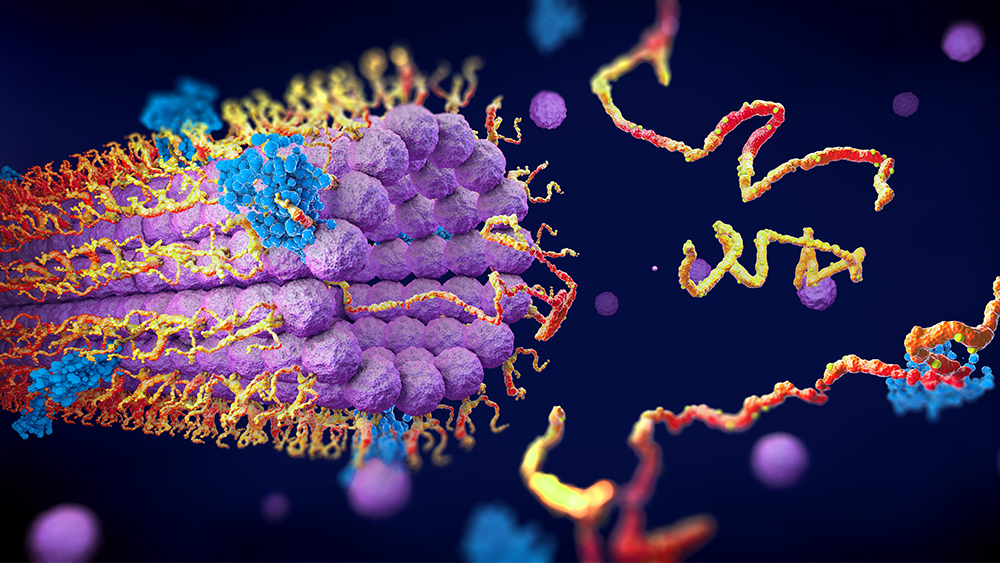
Recently, scientists at Tohoku University in Japan have discovered some aspects of a submicroscopic machine called a transport protein.6 It’s called secretory pathway Ca2+/Mn2+-ATPase, or SPCA for short, and is found in the Golgi apparatus (a series of flattened membranes in the eukaryotic cell designed to package products of cell metabolism). SPCA is involved with calcium transport which is critical for cell signaling (i.e., signal transduction), and it aids in proper protein function. But SPCA is also involved in preventing the accumulation of manganese that would otherwise build to toxic levels.
The researchers delved into SPCA’s structure in order to determine its function, particularly in regard to the inherited condition called Hailey-Hailey disease, characterized by patches of blisters in skin folds. Such a condition arises due to a mutation in the gene that codes for SPCA.
The biologists succeeded in mapping “where mutations on the protein can cause functional defects and eventually lead to Hailey-Hailey disease.”6
Cryo-electron microscopy studies samples frozen at very low temperatures, with the protein molecular motions [of SCPA] immobilized. Proteins normally move and change shape as they go about their normal functions, but whatever the microscope captures can only reveal one specific state. The Tohoku study used the technique to elucidate three of the protein's states: as it is bound to calcium and manganese ions, as it is bound to the energy-providing molecule ATP, and in its metal-unbound phosphorylated state. So it gave three snapshots of states the protein would normally switch between.6
A picture was provided of the Hailey-Hailey disease-associated mutations within the highly folded SCPA protein molecule. Even a slight misfolding of a protein—particularly an enzyme with critical active sites—can have devastating results for the organism. This precise protein folding continues to be an enigma for evolutionists. Two biochemists could only say, “Protein structures [via folding] have evolved to function in particular cellular environments.”7 But “have evolved” is hardly a scientific explanation.
How did the SCPA protein, as well as millions of other proteins, fold? For example, about one out of every ten unfolded polypeptides require a designed nanostructure called TRiC, which is a tube designed with a complex lid on each end.
The unfinished protein is brought briefly inside TRiC, where forces work on rapidly folding the raw protein in ways that are currently unknown. This could be seen as the magician’s rabbit-in-the-hat trick, but is clearly more mysterious, mind-boggling, and molecular (tiny)! TRiC discoverer Judith Frydman, an associate professor of biology at Stanford University, said in a classic understatement, "It is a very complex mechanism."8
The traditional evolutionary portrayal of life developing through chance, time, and natural processes crumbles at every level,9 particularly submicroscopically. The cryo-electron structure of SCPA “has provided many important insights [but] it is not enough to describe the whole picture.”6 The whole picture of protein structure, protein function, and intricate processes at the cellular level really do show that "the invisible things of him from the creation of the world are clearly seen.”10
References
1. Psalm 19:12. Psalm 139:14
3. Alberts, B. et al. 2022. Molecular Biology of the Cell. 7th edition. New York, NY: W.W. Norton and Company.
4. Thomas, B. DNA Repair Enzymes: Vital Links in the Chain of Life. Creation Science Update. Posted on ICR.org August 27, 2008, accessed March 24, 2023.
5. Sherwin, F. DNA Paramedics Repair Chromosomes. Creation Science Update. Posted on ICR.org July 24, 2018, accessed March 24, 2023.
6. Inaba, K. “Cryo-Electron Microscopy Captures Structure of a Protein Pump.” Tohoku University Global Site. Posted on tohoku.ac.jp March 24, 2023, accessed March 24, 2023.
7. Nelson, D. and M. Cox. 2017. Principles of Biochemistry. 7th edition. New York: W.H. Freeman, 143.
8. Sherwin, F. 2008. Evolution Continues to Be a Hard Cell. Acts & Facts. 37 (9): 14.
9. Tomkins, J. In Honor of Darwin’s 200th Birthday: Evolution’s Biggest Gaps. Creation Science Update. Posted on ICR.org March 12, 2009, accessed March 24, 2023.
10. Romans 1:20
* Dr. Sherwin is science news writer at the Institute for Creation Research. He earned an M.A. in zoology from the University of Northern Colorado and received an Honorary Doctorate of Science from Pensacola Christian College.