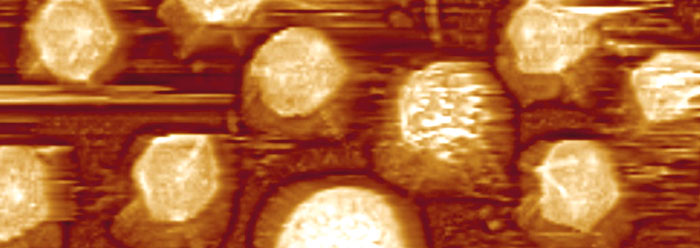
In 1992, researchers discovered a giant virus with so much DNA that some have called it a “viral missing link.” Evolutionary scientists claimed that it has features that reflect both a living cell and non-living matter, and may thus provide a clue as to the origin of the first cell.1 But new high resolution images and a closer inspection of this massive virus actually point to design, rather than evolutionary development.
The Acanthamoeba polyphaga Mimivirus is so rare that it is the only viral species in its genus. Its capsid, or protein shell, is a 400nm-wide icosahedron—a mammoth among viruses. Three-dimensional images of its surface were prepared by cleverly removing the host of protein fibers attached to the capsid, and then patching together thousands of electron micrographs.2 Although there is plenty of space for more, the mimivirus holds quite a bit of DNA inside its capsid. Preliminary DNA sequence comparisons indicated that it has about 905 genes.3
A handful of these genes (lengths of DNA that code for specific proteins) are unique among viruses, and a few were thought to only occur in whole, living cells. Thus, some researchers have interpreted the considerable size of the capsid, the significant amount of DNA, the large number of genes, and the presence of genes thought to be exclusive to cellular life to mean that the presumed evolutionary ancestor to the first living cell may have resembled the mimivirus.
But the mimivirus has no machinery to unpack, transcribe, translate, or repair its genes. Like all other viruses, it borrows pre-existing, fully functioning cellular machinery for that purpose from a living host cell. A mimivirus also doesn’t detect or respond to its environment, reproduce, or perform any metabolic activities on its own. It just passively floats around until it “docks” with a host. The difference between a mimivirus and a host cell is like the difference between a computer disk with a virus on it and a fully-functioning computer (except that unlike computers, living cells manufacture their own parts, and repair and copy themselves).
Rather than serving as developmental stepping stones, viruses may originally have served a specific purpose. Evolutionary-turned-creation scientist Gary Parker proposed in 2006 that “in God’s originally perfect creation, the interlocking of docking and receptor proteins was designed to allow viruses to insert their DNA (or RNA) into only those cells in which gene transfer would be beneficial….Perhaps God, the ultimate Genetic Engineer, designed viruses as gene carriers, especially for bacteria, which are incredibly streamlined for genetic efficiency and rapid response to environmental stimuli.”4
Most viruses are host-specific, and many are even tissue-specific. The mimivirus specifically infects amoebas. Though they are single-celled creatures, amoebas have massive genomes. For example, Amoeba dubia has 200 times more DNA than humans.5 It would make sense that a viral particle that interacts exclusively with amoebas would also have a large DNA content, especially if it was originally designed to carry extra “repair or replace” copies of genes for the amoebas. Thus, the mimivirus does not look at all like a missing link but rather like the remnant of what was once a well-designed, very good aspect of God’s living world.
References
- Keim, B. Viral Missing Link Caught on Film. Wired Science. Posted on wired.com May 5, 2009, accessed May 6, 2009.
- Xiao, C. et al. 2009. Structural Studies of the Giant Mimivirus. Public Library of Science Biology. 7 (4): e92.
- Mimiviridae. Fact sheet from Swiss Institute of Bioinformatics. Posted on expasy.org, accessed May 7, 2009.
- Parker, G. 2006. Creation: Facts of Life. Green River, AR: Master Books, 140.
- Gregory, T. R., and P. D. N. Hebert. 1999. The Modulation of DNA Content: Proximate Causes and Ultimate Consequences. Genome Research. 9 (4): 317-324.
Image Credit: PLoS Biology
* Mr. Thomas is Science Writer at the Institute for Creation Research.
Article posted on May 15, 2009.